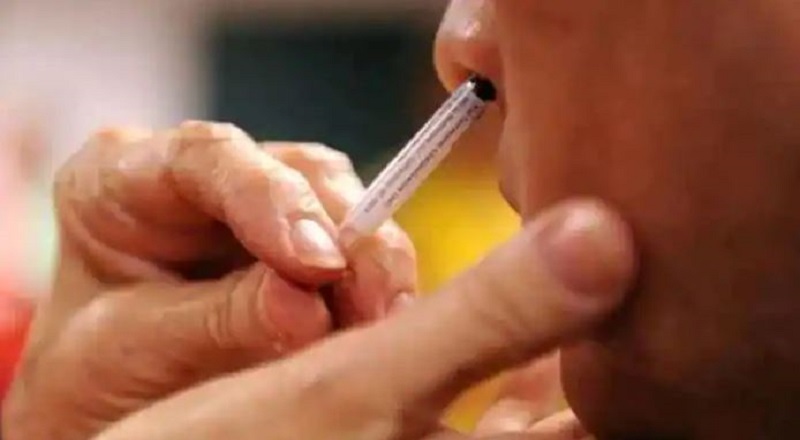
DCGI approves Bharat Biotech’s nasal covid vaccine. Union Health Minister Dr Mansukh Mandaviya said Bharat Biotech on Tuesday received emergency use authorization from the DCGI for the intranasal Covid-19 vaccine.
This is India’s first intranasal vaccine for Covid-19, he said. Union Health Minister Mansukh Mandaviya took to Twitter and expressed great hope for India’s fight against COVID-19. Bharat Biotech’s ChAd36-SARS-CoV-S COVID-19 (chimpanzee adenovirus vectored) recombinant nasal vaccine approved by @CDSCO_INDIA_INF.DCGI approves Bharat Biotech’s nasal covid 19 vaccine
The intranasal vaccine is BBV154, whose technology was acquired by Bharat Biotech from Washington University in St. Louis. It is the first vaccine of its kind to be tested on humans in India.
Last month after completing the phase-III and booster dose trials for its intranasal Covid-19 vaccine, Bharat Biotech said it had conducted two separate trials for its intranasal Covid vaccine, one as a primary dose schedule and another as a booster dose, for subjects who have been double vaccinated with the two commonly administered Covid vaccines in India.
It has proven to be safe, well-tolerated and immunogenic in subjects in controlled trials, BBIL said in a statement.The data from both Phase III human clinical trials have been submitted for approval to national regulatory authorities, the company said.
Also Read: CBSE Class 10, class 12 compartment results 2022: cbse.gov.in download
Also Read: China Earthquake: 6.6 Magnitude Strong Earthquake in China; 46 people died, 30 people were injured
“Under the leadership of Prime Minister Narendra Modi, India has harnessed its science, R&D and human resources in the fight against COVID-19. With a science-driven approach and Sabka Prayas, we will defeat COVID-19,” he said. This will further strengthen India’s collective fight against the pandemic, the health minister said.

Comments are closed.